
It is a rapid and intense onset of withdrawal symptoms initiated by medication as part of addiction treatment. In the case of buprenorphine, because it has a higher binding strength at the opioid receptor, it competes for the receptor, “kicks off” and replaces existing opioids. If a significant amount of opioids are expelled from the receptors and replaced, the opioid physically dependent patient will feel the rapid loss of the opioid effect, initiating withdrawal symptoms. More precisely, precipitated withdrawal can occur when an antagonist (or partial agonist, such as buprenorphine) is administered to a patient with a physical dependence on full agonist opioids. Due to the high affinity but low intrinsic activity of buprenorphine at the µ-receptor, the partial agonist displaces full agonist opioids from the µ-receptors, but activates the receptor to a lesser degree than full agonists which results in a net decrease in opioid agonist effect, thereby precipitating withdrawal.¹
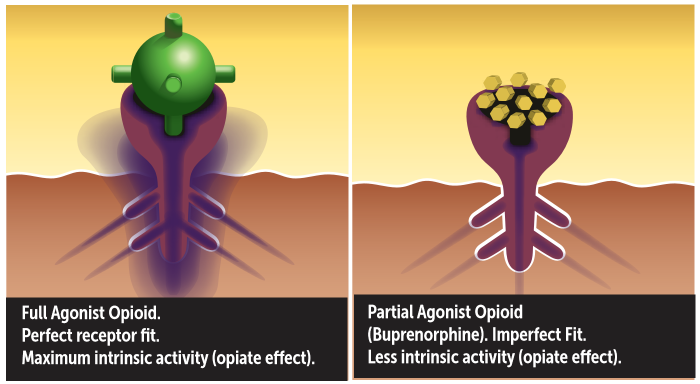
A common misconception is that the naloxone in the buprenorphine/naloxone combination medication initiates precipitated withdrawal. Naloxone may only initiate withdrawal if injected into a person physically dependent on opioids. Taken sublingually, as directed, naloxone is clinically insignificant and has virtually no effect. (Except in rare cases of an allergic reaction or naloxone hypersensitivity.²)
AVOIDING PRECIPITATED WITHDRAWAL
 Patient education and developing realistic expectations are essential before beginning a drug addiction treatment program. To avoid precipitated withdrawal, physically dependent patients must no longer be experiencing the agonist effects of an opioid. One way to gauge this is to observe objective symptoms of withdrawal sufficient to score a minimum of 5 to 6 on the COWS (Clinical Opioid Withdrawal Scale). Scores of >10 are preferable. Due to patient individuality, required abstinent times may vary considerably from patient to patient. Only use the time since last substance use as an estimate to anticipate the onset of withdrawal symptoms.? The induction begins by assessing last use of all opioids, short and long-acting, objective and subjective symptoms, and a COWS score calculation. If not in sufficient withdrawal (mild to moderate: COWS of 5 to 24), it is in the patient’s best interest to wait. Long-acting opioids will require a longer period of abstinence, than short-acting opioids.
Patient education and developing realistic expectations are essential before beginning a drug addiction treatment program. To avoid precipitated withdrawal, physically dependent patients must no longer be experiencing the agonist effects of an opioid. One way to gauge this is to observe objective symptoms of withdrawal sufficient to score a minimum of 5 to 6 on the COWS (Clinical Opioid Withdrawal Scale). Scores of >10 are preferable. Due to patient individuality, required abstinent times may vary considerably from patient to patient. Only use the time since last substance use as an estimate to anticipate the onset of withdrawal symptoms.? The induction begins by assessing last use of all opioids, short and long-acting, objective and subjective symptoms, and a COWS score calculation. If not in sufficient withdrawal (mild to moderate: COWS of 5 to 24), it is in the patient’s best interest to wait. Long-acting opioids will require a longer period of abstinence, than short-acting opioids.
SHORT-ACTING OPIOIDS
(HEROIN, CRUSHED OXYCONTIN®, PERCOCET®, VICODIN®, OXYCODONE, AND OTHERS)
Prior to induction, patients must abstain from all short-acting opioids for 12 to 24 hours and/or have objective withdrawal symptoms sufficient to produce a score of five to 24 on the COWS.¹
LONG-ACTING OPIOIDS
OXYCONTIN® (TAKEN ORALLY)
Discontinue use for at least 24 hours prior to induction. A minimal score of at least five on the COWS is recommended, although some physicians prefer scores of 15 or higher.?
METHADONE
It is recommended that patients transitioning from methadone to buprenorphine slowly taper to 30 mg/day of methadone for at least one week. The last dose must be no less than 36 hours prior to induction and may be 96 hours or more. A minimal score of at least five on the COWS is recommended, although some physicians prefer scores of 15 or higher.?
Patients transferring from methadone or another long-acting opioid to buprenorphine treatment may experience discomfort for several days and dysphoria for up to two weeks.³ The goal of induction is to safely suppress opioid withdrawal as rapidly as possible with adequate doses of buprenorphine. Failure to do so may cause patients to use opioids or other medications to alleviate opioid withdrawal symptoms or may lead to early treatment dropout.³ To achieve this, some physicians have found they may need to dose as high as 32 mgs the first day with some methadone to buprenorphine transfers.?
MEDICATION-ASSISTED TREATMENT AT BRIGHTVIEW
BrightView offers medication assisted treatment at convenient addiction treatment center locations throughout Ohio. Our locations include:
- Cincinnati addiction treatment center
- Dayton addiction treatment center
- Columbus East addiction treatment center
- Columbus West addiction treatment center
- Toledo addiction treatment center
- Mason addiction treatment center
Connect with our team online today to learn more.
¹ Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. http://naabt.org/links/TIP_40_PDF.pdf
² FDA. Full Prescribing information on Subutex® (buprenorphine)/Suboxone® (buprenorphine/naloxone) www.fda.gov/cder/foi/label/2002/20732lbl.pdf
³ Dosing Guide Maintenance therapy for Opioid Dependence. Suboxone®/Subutex® www.suboxone.com/pdfs/DosingGuides.pdf
? Practical Considerations for the use of Buprenorphine Hendrée E. Jones, Ph.D., Johns Hopkins University School of Medicine, Baltimore, MD
? Physician Clinical Support System: www.pcssmentor.org Transfer from Methadone to Buprenorphine, Paul P. Casadonte, MD, PCSS guidance paper. 8/9/2006 http://www.pcssmentor.org/pcss/documents2/PCSS_MethadoneBuprenorphineTransfer.pdf